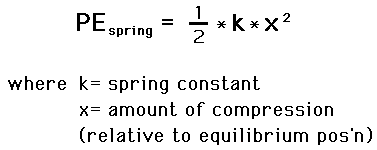Chemical energy
Chemical energy is basically the enrgy that is stored in molecular bonds, for example carbohydrate or the food we eat everyday. When a chemical reaction occurs, it converts other types of energy into chemical energy. Releasing chemical energy means that the energy from the molecular bonds is released, this is an exotermic chemical reaction. Absorbing chemical energy means that more energy is stored in bonds of molecules. In another sense, chemical energy is also stored energy, or potential energy.
The process of photosynthesis converts light energy into chemical energy in carbohydrate molecules. Living organisms like you and me use this form of energy for food and metabolism. Yet, this energy is usually stored in the bonds of our molecules and compounds. Over a long period of time, this stored energy is released by living cells by a process called respiration. When this occurs, chemical energy is again converted into other types of energy, such as kinetic energy used for movement, or heat energy to keep our body warm.
Gravitational Potential Energy
 For all objects on Earth, gravitatonal potential energy acts on them because it is postioned within the gravitational field. Therefore, when an object is elivated at certain height, we can say that there are gravitational potential energy stored in the object because the energy is stored within the object and has the potential to be released. The potential energy at height h is equal to the work required to elivate the object. Since the force required to lift the object is equal to its weight, therefore gravitational potential energy is equal to weight times the height. In a simple example, if you lift an object, such as your shoe to a certain height, work is being done because a force was applied and there was a displacement. Now you've lost some energy having to lift the shoe, but we know that energy cannot be destroyed or created. Therefore, the chemical energy you used which was converted into kinetic energy in the movement of muscles or finally converted into gravitational potential energy. Once you let go of the shoe, the shoe would fall wouldn't it? Then again the gravitational potential energy stored in the shoe is again converted back into kinetic energy.
For all objects on Earth, gravitatonal potential energy acts on them because it is postioned within the gravitational field. Therefore, when an object is elivated at certain height, we can say that there are gravitational potential energy stored in the object because the energy is stored within the object and has the potential to be released. The potential energy at height h is equal to the work required to elivate the object. Since the force required to lift the object is equal to its weight, therefore gravitational potential energy is equal to weight times the height. In a simple example, if you lift an object, such as your shoe to a certain height, work is being done because a force was applied and there was a displacement. Now you've lost some energy having to lift the shoe, but we know that energy cannot be destroyed or created. Therefore, the chemical energy you used which was converted into kinetic energy in the movement of muscles or finally converted into gravitational potential energy. Once you let go of the shoe, the shoe would fall wouldn't it? Then again the gravitational potential energy stored in the shoe is again converted back into kinetic energy.Elastic Potential Energy
When an elastic material, such as a spring is stretched, we're again storing potential energy in the spring. Other elastic materials, such as rubber band, trampolines, bows and arrows all can store potential energy when they're stretched from its original form.Furthermore, it's only common sense that the more we stretch, the more potential energy is stored.
When there are no potential energy stored in an elastic, we say that its at rest or its equilibrium position. In this position, no force is applied to to stretch the elastic to store potential energy in them.How we measure the relative elastic potential energy stored within an elastic is by how much it is stretched from its equilbrium position, formed by equation:
Mechanical Kinetic Energy
Whenever work is done by force, whether supplied from energy such as chemical energy from food or other sources of potential energy, the object work is done upon acquires mechanical energy. Thus mechanical energy can be both kinetic or potential energy. In other words, mechanical energy can be resulted from an object's motion (kinetic energy) or its stored energy position (potential energy). Think about car engines, pulleys, cannons, all these things are machines used to do work. Therefore, another way of looking at mechanical energy is the energy that supplies an object to do work. Since energy cannot be destroyed or created, mechanical energy must be first be converted from another energy source. Think about a pitcher in a baseball game, when the ball is pitched, it is doing work by travelling a distance at a high velocity. The mechanical energy in the ball however, was converted from the kinetic energy from the pitcher's arm muscles.
Another illustration of mechanical energy:
 Thermal energy comes from heat produced by any kind of source, such as fire. This form of energy is created due to the increased activity or velocity of molecules in a substance. We generally feel thermal energy as something that is too cold or too hot for our comfort. This is because the velocity of the movement of molecules of the substance is either moving too fast or too slow for our body's comfort. Temperature however, is a relative measure of the amount of kinetic energy stored in molecules in a substance. For thermal energy, again the laws of conservation of energy applies. The laws of thermodynaics explains that thermal energy can be exchanged from one object to another.For instance, heating up a bucket of water will cause the water molecules to faster and thus boil.However, this energy that water acquired over the period of heating was not created, it was received from the energy of the heater, which lost the same amount of energy that water gained.
Thermal energy comes from heat produced by any kind of source, such as fire. This form of energy is created due to the increased activity or velocity of molecules in a substance. We generally feel thermal energy as something that is too cold or too hot for our comfort. This is because the velocity of the movement of molecules of the substance is either moving too fast or too slow for our body's comfort. Temperature however, is a relative measure of the amount of kinetic energy stored in molecules in a substance. For thermal energy, again the laws of conservation of energy applies. The laws of thermodynaics explains that thermal energy can be exchanged from one object to another.For instance, heating up a bucket of water will cause the water molecules to faster and thus boil.However, this energy that water acquired over the period of heating was not created, it was received from the energy of the heater, which lost the same amount of energy that water gained.Sound Energy
When we pluck the strings on a guitar, or smack the surface of a drum, we hear a sound or music if we're really good at playing these instruments. In turn, we have also created a type of energy as we hear the music. Sound energy is the energy produced by vibrations travelling through the environment, or a medium. This vibration causes waves of pressure which lead to some level of compression and rarefaction in the environment that it's travelling in. So one may ask: what type of energy is sound energy? Well, it's obviously not chemical energy since the energy is not stored in any bonds of molecules. It's not gravitational energy nor thermal energy. Becauuuuuuuuuuuuuse: sound is a form of MECHANICAL energy! However, sound energy is not used to do the amount of work human requires from machine because the amount of energy gained from sound is usually very small.